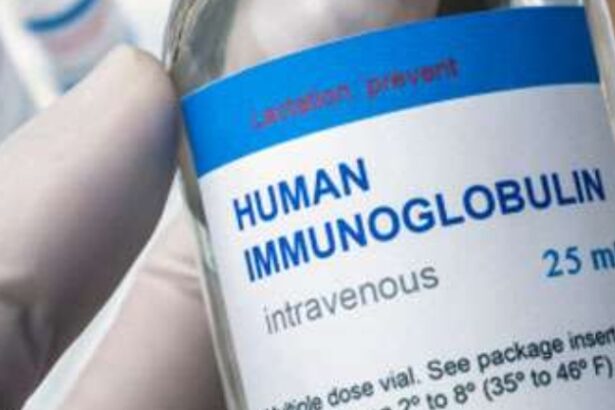
The National Medicines Regulatory Authority (NMRA) has assured that no substandard immunoglobulin is currently in use at government hospitals, contrary to recent media reports.
In a statement, NMRA Chairman Dr. Ananda Wijewickrema said the immunoglobulin in question was manufactured by ICHOR Biologics Pvt. Ltd. in India and received regulatory approval in 2023.
He noted that the medicine was initially flagged following a complaint after use, and concerns were raised during testing. As a precaution, the Medical Supplies Division was instructed to halt its distribution.
Subsequent testing, including analysis by India’s Central Drugs Standard Control Organisation, confirmed that the product met required standards. Based on these findings, a special technical committee of specialist doctors approved its continued use.
Dr. Wijewickrema said there should be no doubts about the medicine’s quality, as it had undergone all necessary evaluations and received appropriate approvals. (Newswire)
The post NMRA assures no substandard Immunoglobulin in use appeared first on Newswire.